

Vendor Audit
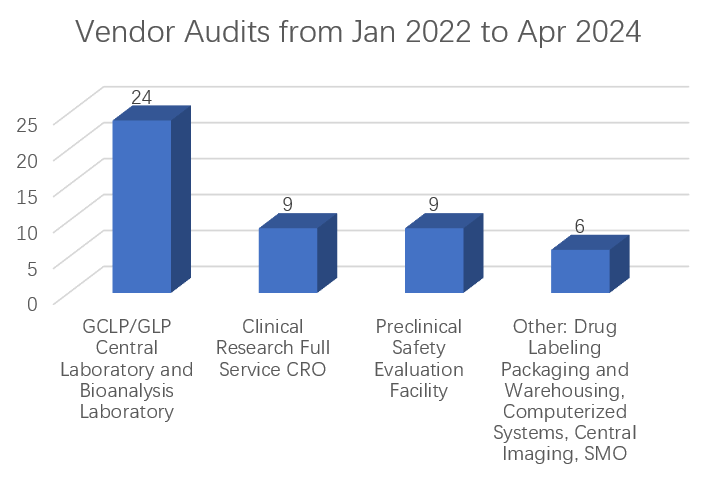
From January 2022 to February 2024, we performed 48 GxP vendor audits.
Including but not limited to nine (9) for clinical research full service CRO (GCP), nine (9) for preclinical safety evaluation facility (GLP), 24 for GCLP/GLP central laboratory and bioanalysis laboratory, and other types of vendors like drug labeling packaging and warehousing, computerized systems, central imaging, SMO, etc. (GCP/GSP/GMP)
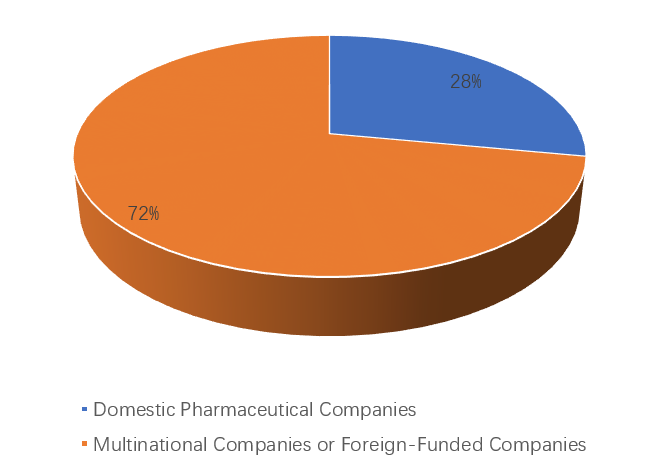
Among them, 28% of the sponsors were domestic pharmaceutical companies, while 72% were multinational companies or foreign-funded companies
06-08-2024